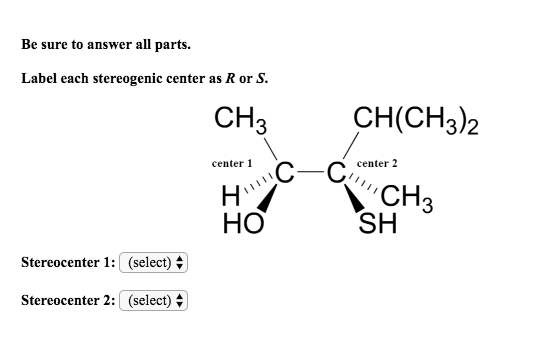
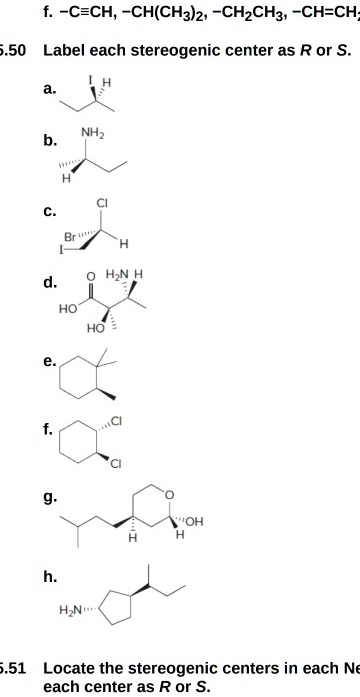
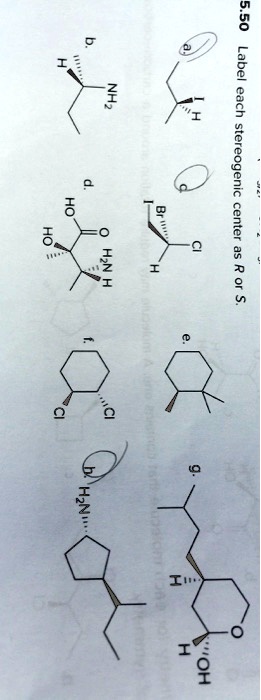
45 label each stereogenic center as r or s
Finding R and S for Chiral Centers - Organic Chemistry | Socratic R and S are labels assigned to the stereocenters of a molecule. To easily find the R and S centers, label the four bonded molecules 1 to 4 in order of atomic number. Place the 4 molecule in the back of the chiral center and then in a clockwise (R) or counterclockwise (S) direction label the bonds with atoms 1, 2, 3. R/S - Two Stereogenic Centers The R/S Naming System Two or more Stereogenic Centers Optical Activity R/S Naming Diastereoisomerism Meso Compounds Today, we'll look at naming compounds with stereocenters, and then we'll examine the complications which arise when a molecule has more than one stereocenter in it.
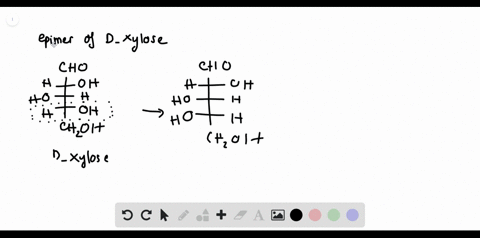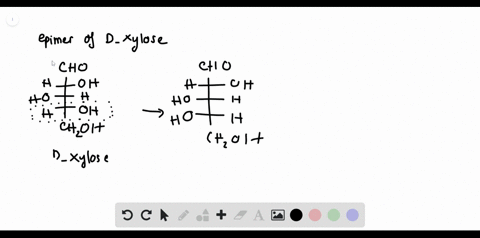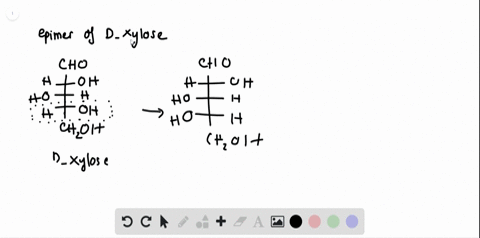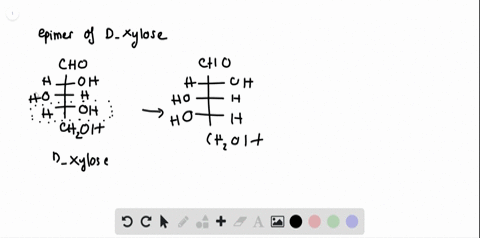
SOLVED:Label each stereogenic center as R or S. this question asks us to aside, as are our configuration for Glucose s. So this is me glucose. Um, again gonna start by assigning the, um ah. Parity of the group sold is this one? This is two. This is three and Hodgins on this side, so we have to reverse the order. So 123 is cattle. Clockwise prefers is, huh? This is one again. Appears to marry is three.
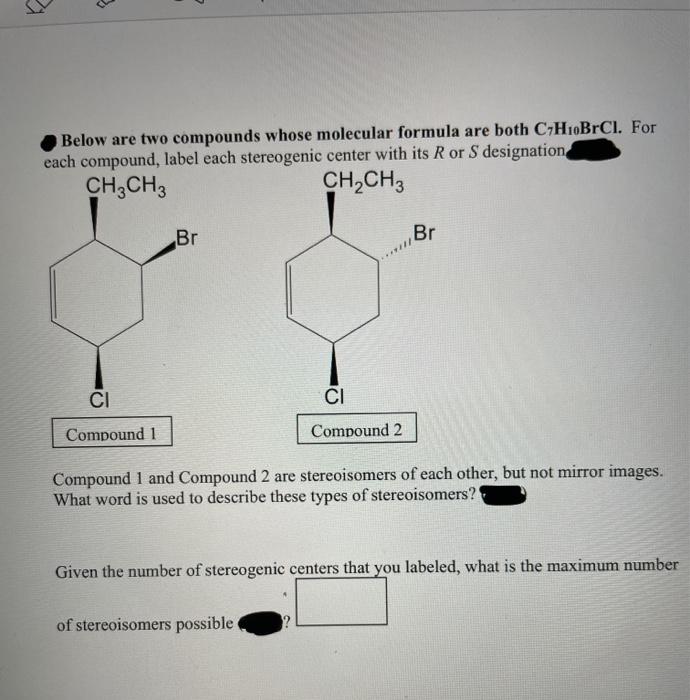
Label each stereogenic center as r or s
Stereochemistry configuration of r and s - slideshare.net It labels each chiral center R or S according to a system by which its substituents are each assigned a priority, according to the Cahn-Ingold-Prelog priority rules (CIP), based on atomic number. ... stereogenic center, the higher the atomic mass, the higher the rank. 2. If two or more atoms directly attached to the stereogenic center have ... Answered: Designating a Stereogenic Center as R… | bartleby Solution for Designating a Stereogenic Center as R or S When the Lowest-Priority Group Is Not Drawn Back Label each stereogenic center as Ror S. а. OH b. OH How to Determine the R and S configuration - Chemistry Steps Step 1: Give each atom connected to the chiral center a priority based on its atomic number. The higher the atomic number, the higher the priority. So, based on this, bromine gets priority one, the oxygen gets priority two, the methyl carbon is the third and the hydrogen is the lowest priority-four: Step 2:
Label each stereogenic center as r or s. Solved: Chapter 5 Problem 47P Solution - Chegg Rule 3: If the first atom of each substituent is same then give priority to the second atom in each substituent. This process continues to the third and fourth atom until the rule difference is reached. Rule 4: If the substituents have multiple bonds, the multiple bonded atoms are considered as same number of single bonded atoms. Stereochemistry configuration of R and S - SlideShare It labels each chiral center R or S according to a system by which its substituents are each assigned a priority, according to the Cahn-Ingold-Prelog priority rules (CIP), based on atomic number. ... stereogenic center, the higher the atomic mass, the higher the rank. 2. If two or more atoms directly attached to the stereogenic center have ... SOLVED:Label each stereogenic center as R or S. - Numerade let's label each stereo centers are s for part A iodine has the highest priority than the Ethel on the metal than hydrogen. But the lowest priority back, which is hydrogen. And if we move around the ring me of iodine, Ethyl carbon. This is the counter clockwise direction. PDF Priority Rules for Naming Chiral Centers - The R,S System atomic number of the atom that is bonded directly to the chiral center. The higher the atomic number, the higher the priority.! Number the four atoms, or groups of atoms, such that "1" has the highest priority and "4" has the lowest priority. 2. If two or more of the atoms that are bonded directly to the chiral center are the same, then
SOLVED:Label each stereogenic center as R or S. - Numerade This is three. And yeah, for sure. Heterogeneous far. And because hydrogen over here, so we have to reverse. So 123 This is cock wise. Realizes this is s This is one. This is two. This is three, and this is fall. So one, 23 It is, uh, also, uh, car twice. And because Hodgins on this exists, this is s we have to revisit. EOF HOW TO: Assigning chiral centers (R or S) If my fingers follows the direction of 1, 2, and 3, then the chiral center is R. This is easy to remember (right = R). If my right hand does not follow the direction of 1, 2, and 3, then the chiral center is S. 2) Connect the groups in the order: 1, 2, and 3. If the direction is clockwise, then the chiral center is R. 5.6: Labeling Stereogenic Centers with R or S If, however, the arrow points clockwise, ( Right when leaving the 12 o' clock position) then the stereocenter is labeled R ("Rectus" → Latin= "right"). The R or S is then added as a prefix, in parenthesis, to the name of the enantiomer of interest. Example 1 ( R )-2-Bromobutane ( S )-2,3- Dihydroxypropanal
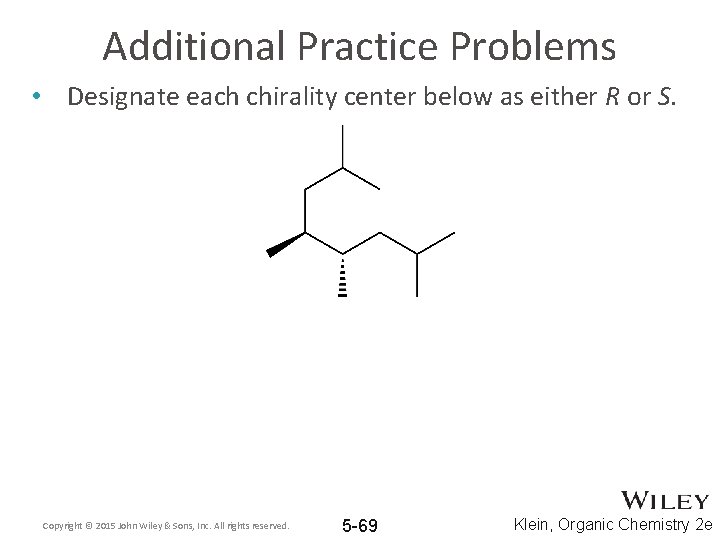
Solved Label each stereogenic center as R or S. | Chegg.com Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 96% (80 ratings) Transcribed image text: Label each stereogenic center as R or S. Previous question Next question. Answered: Label each stereogenic center as R or S… | bartleby The stereogenic center in the given compound has to be mentioned, Q: C) Label each asymmetric carbon in the compound below as R or S. H3C CH2CH2CI H3C CHO ⊙ Draw semicircle goes from 1→2→3 then check clockwise/anticlockwise rotation and 4th priority… How to Assign R / S Configurations to Chiral Centers - dummies Step 1: Prioritizing the substituents. The first step is to prioritize all the substituents from one to four. Bromine is the atom with the largest atomic number, so this substituent is given the highest priority; hydrogen has the smallest atomic number, so it's given the lowest priority. Chlorine gets the number-two priority because it has a ... Draw the eight constitutional isomers having the molecular f | Quizlet C5H11Cl. a. Give the IUPAC name for each compound (ignoring R and S designations). b. Label any stereogenic centers. c. For each constitutional isomer that contains a stereogenic center, draw all possible stereoisomers, and label each stereogenic center as R or S.
Solved: Label each stereogenic center as R or S. | Chegg.com Organic Chemistry (4th Edition) Edit edition Solutions for Chapter 28 Problem 3P: Label each stereogenic center as R or S. … Solutions for problems in chapter 28 1P
Answered: Problem 5.27 (a) Label the four… | bartleby Solution for Problem 5.27 (a) Label the four stereogenic centers in sorbitol as R or S. (b) How are sorbitol and A related? (c) How are sorbitol and B related?…
How to Determine the R and S configuration - Chemistry Steps Step 1: Give each atom connected to the chiral center a priority based on its atomic number. The higher the atomic number, the higher the priority. So, based on this, bromine gets priority one, the oxygen gets priority two, the methyl carbon is the third and the hydrogen is the lowest priority-four: Step 2:
Answered: Designating a Stereogenic Center as R… | bartleby Solution for Designating a Stereogenic Center as R or S When the Lowest-Priority Group Is Not Drawn Back Label each stereogenic center as Ror S. а. OH b. OH
Stereochemistry configuration of r and s - slideshare.net It labels each chiral center R or S according to a system by which its substituents are each assigned a priority, according to the Cahn-Ingold-Prelog priority rules (CIP), based on atomic number. ... stereogenic center, the higher the atomic mass, the higher the rank. 2. If two or more atoms directly attached to the stereogenic center have ...
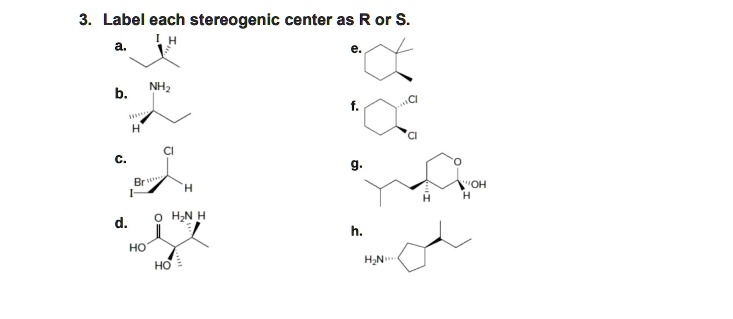
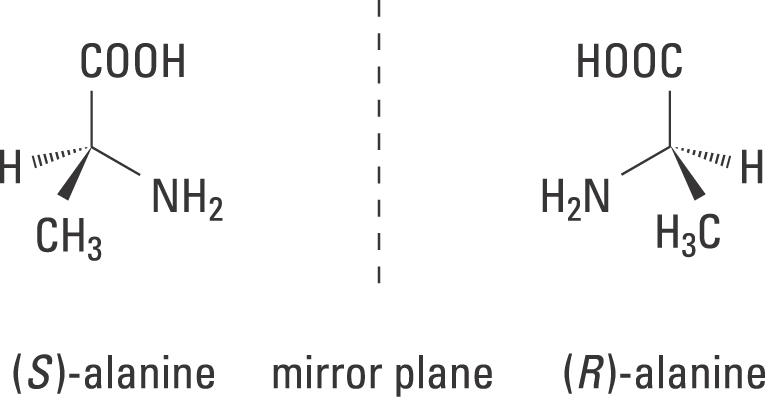

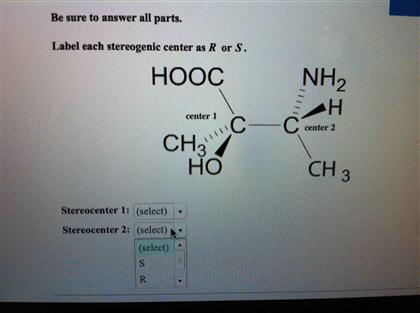



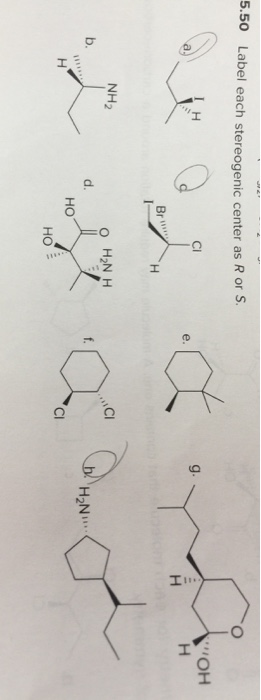


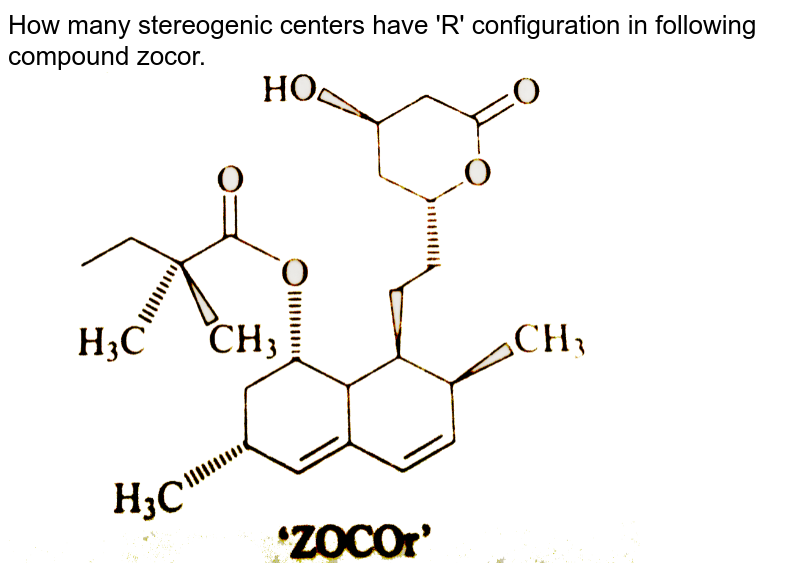
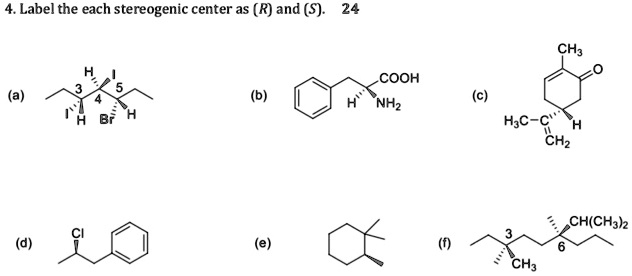

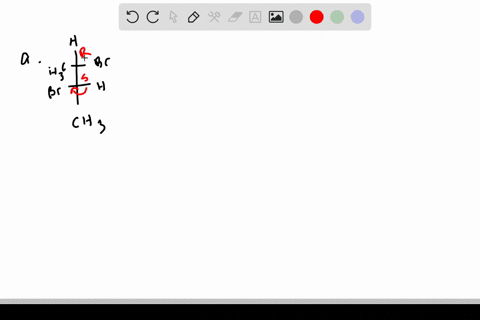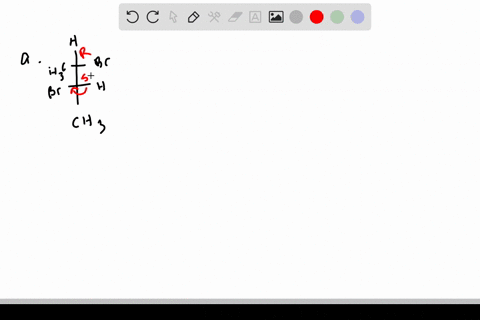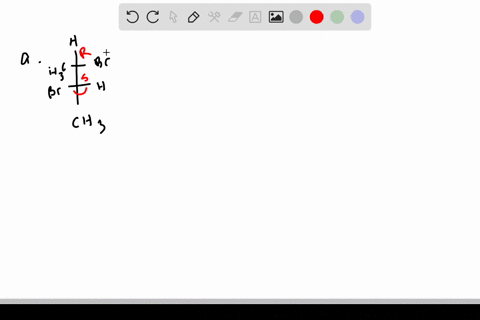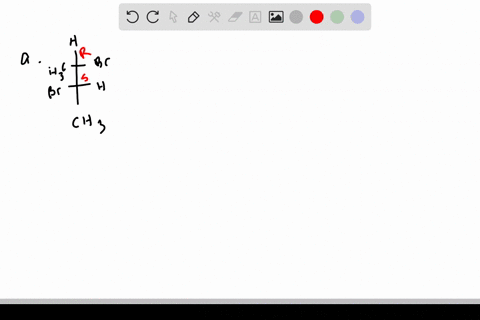
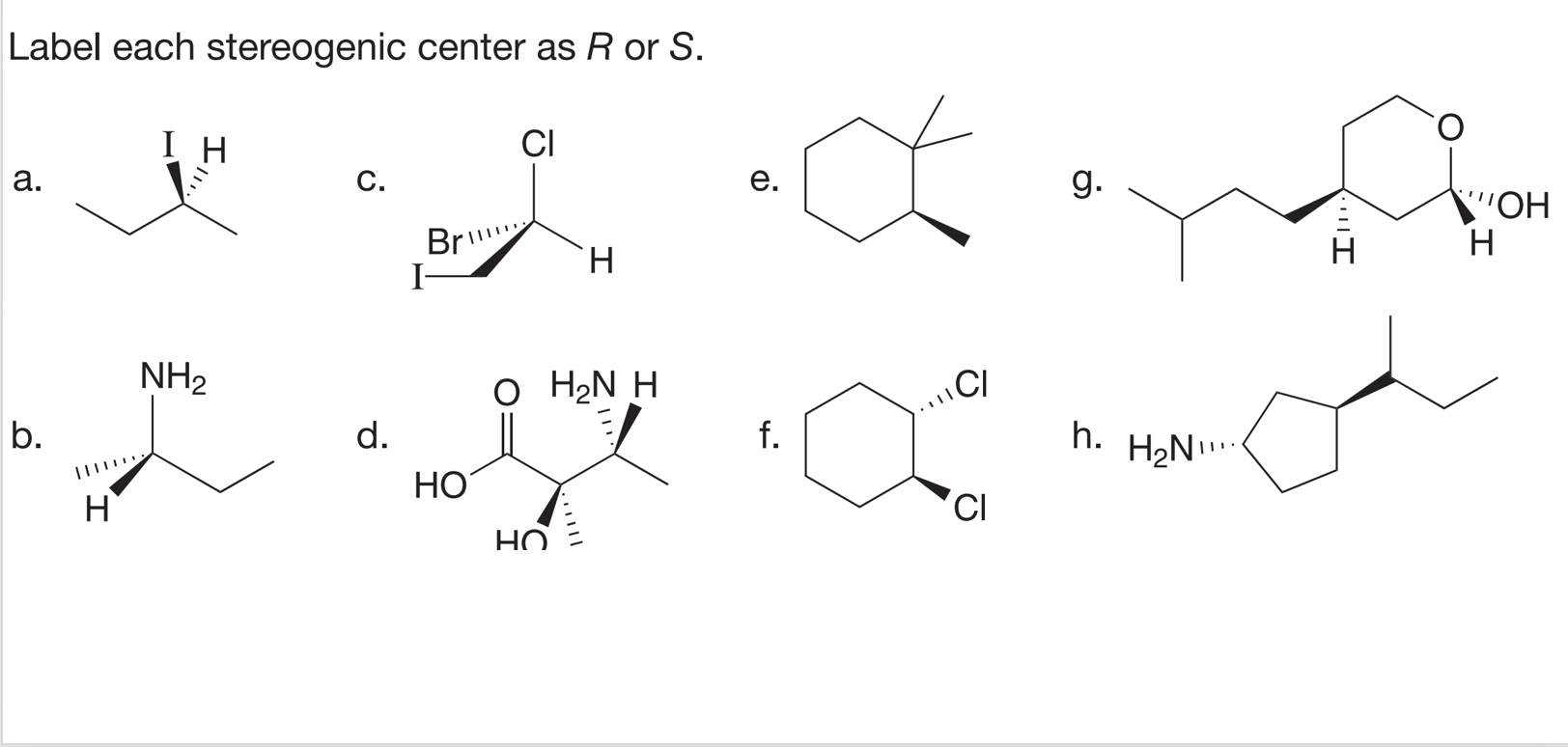
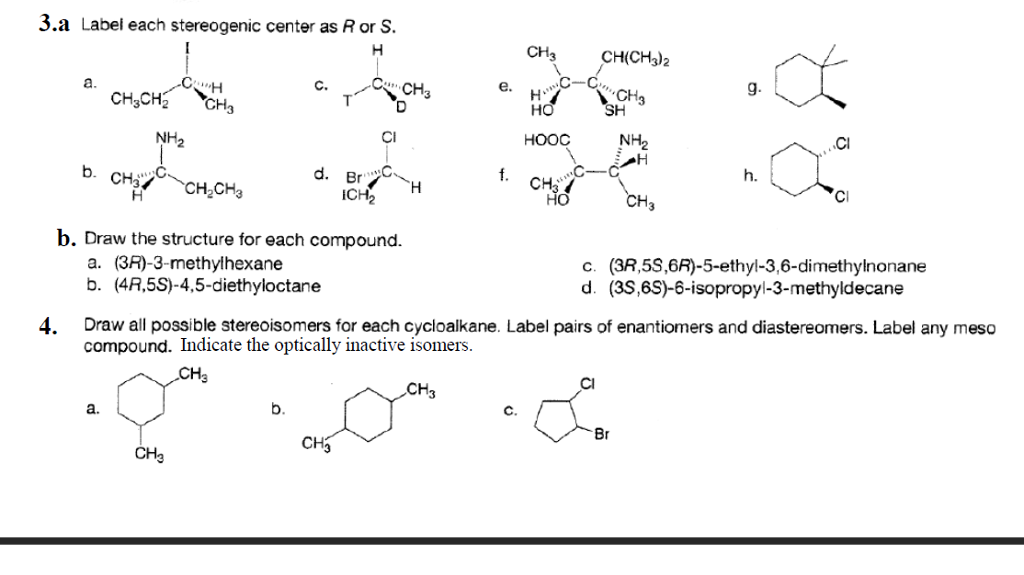
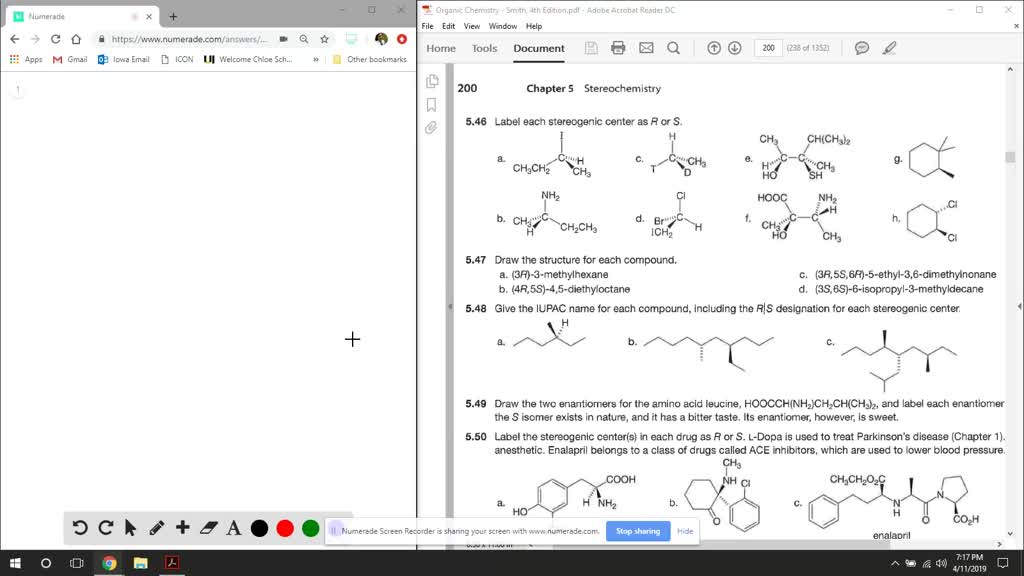
![Solved]: I Label each stereogenic center as R or S. H e. C](https://media.cheggcdn.com/study/6fa/6fa08e1e-4577-44de-ad46-9724b029dc21/image)


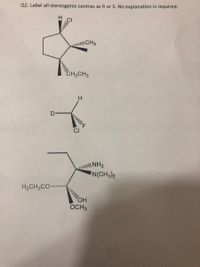
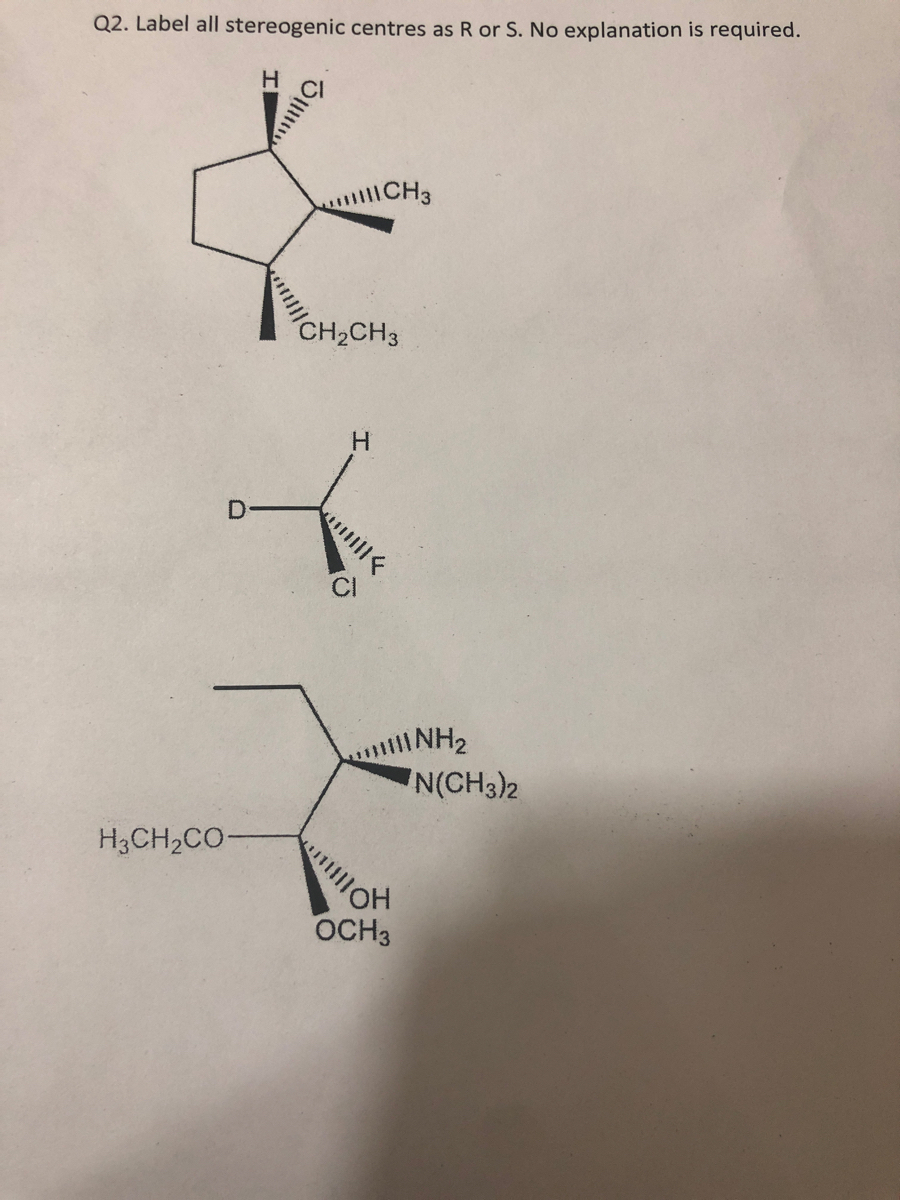
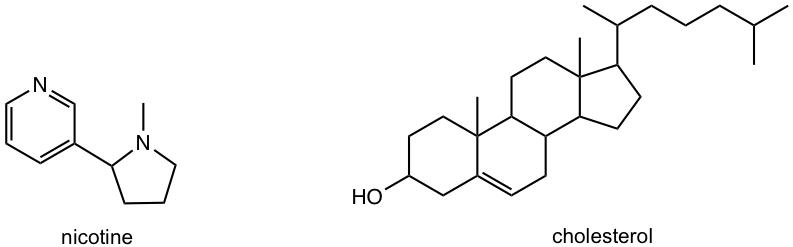
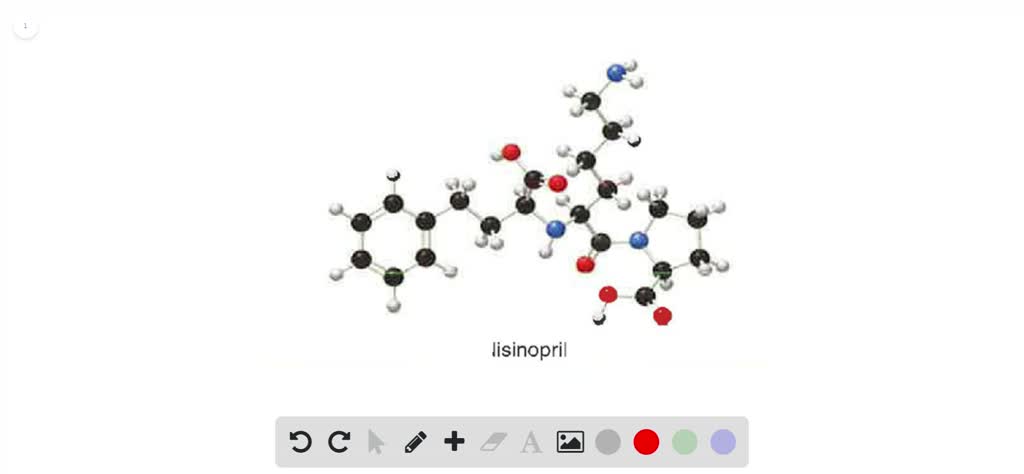

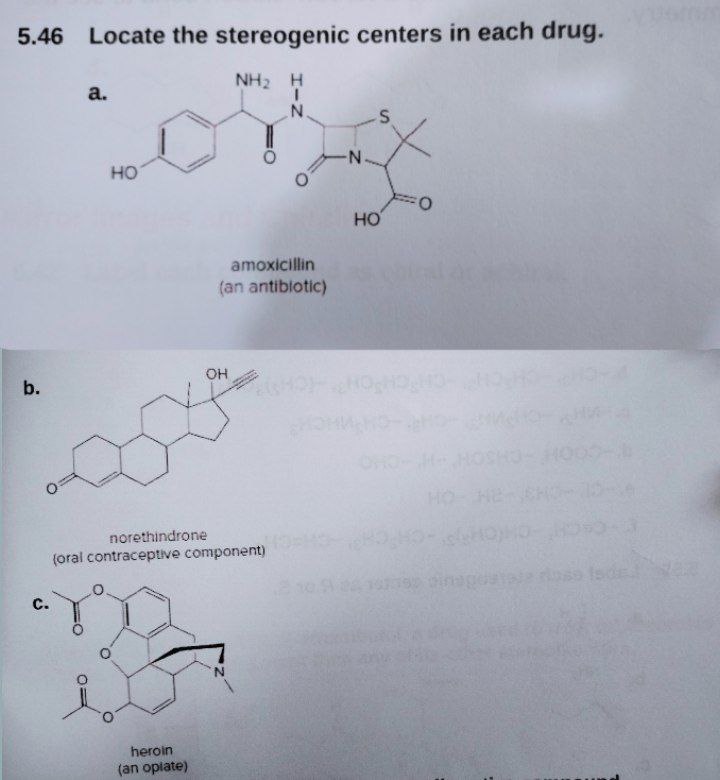


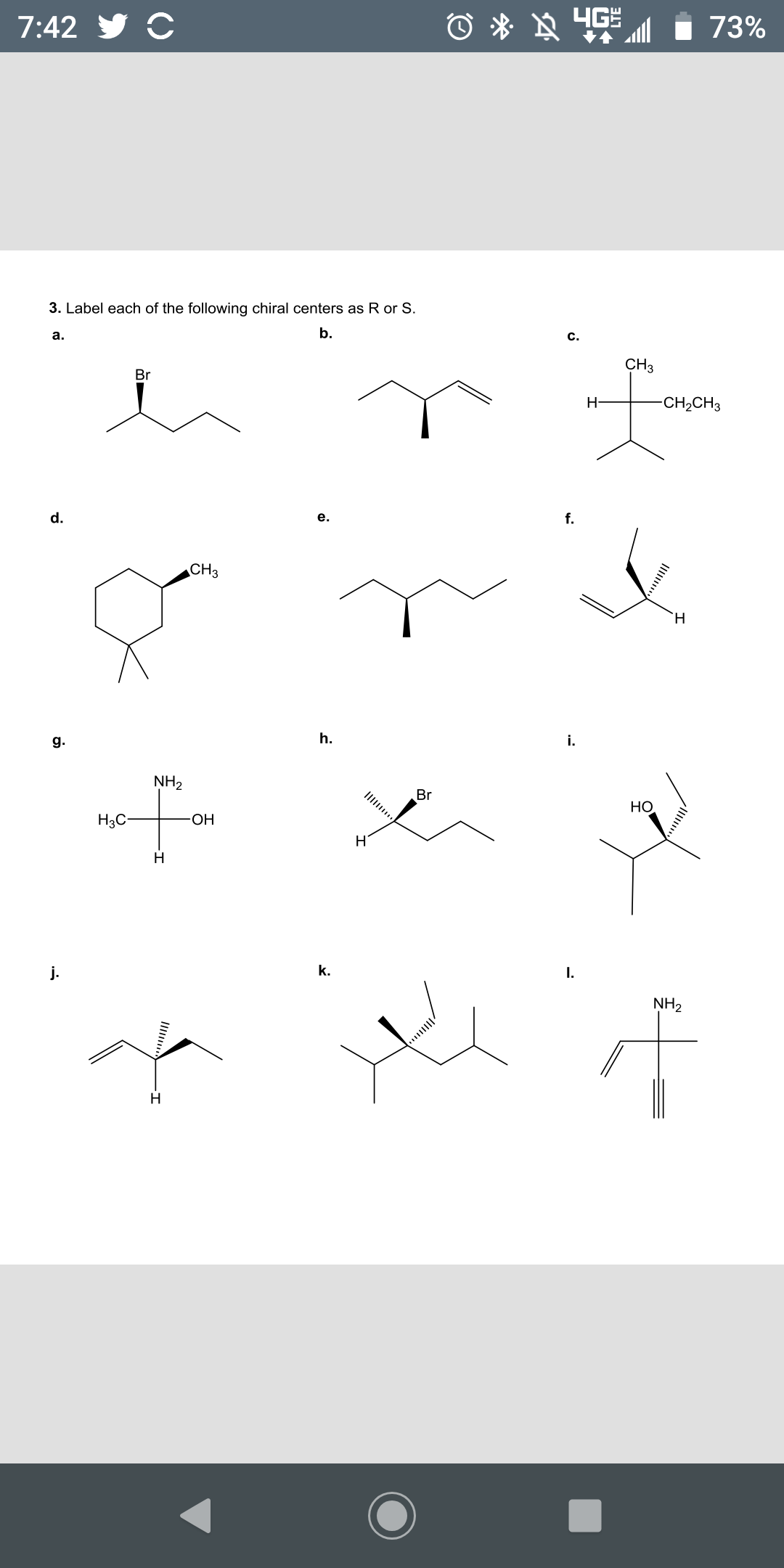
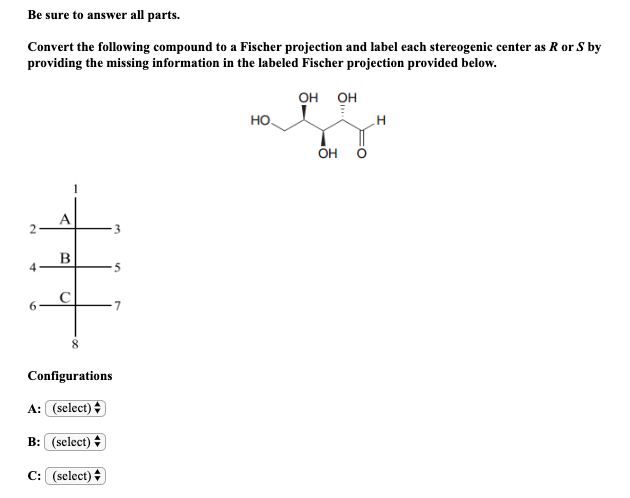
![Solved]: Label the stereogenic centers as S or R. Kindly sh](https://media.cheggcdn.com/study/caa/caab8176-a00d-4044-8919-8c8a2f268a98/image)
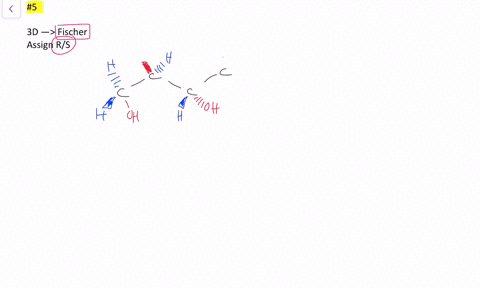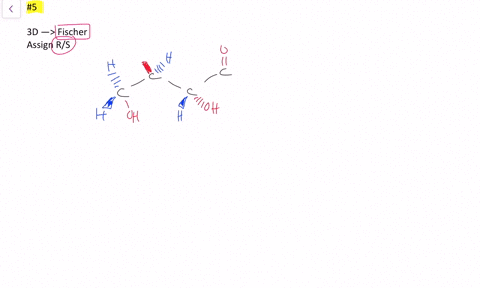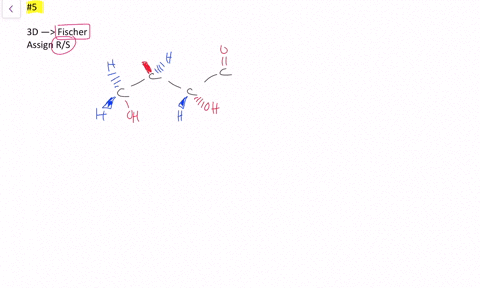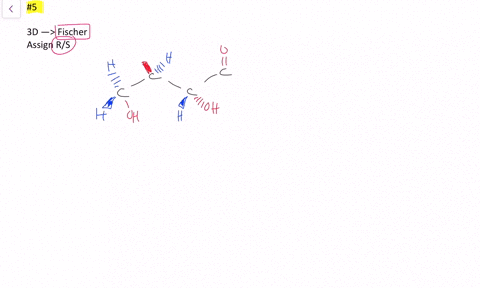
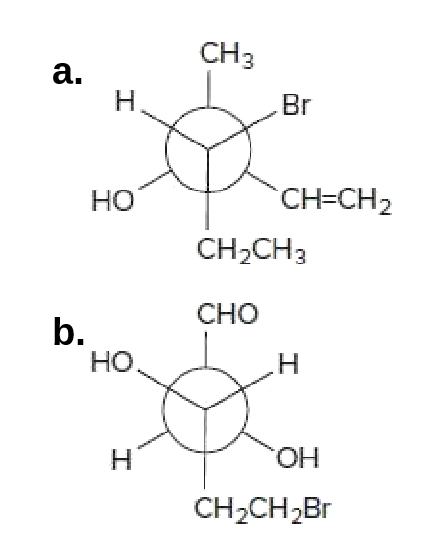
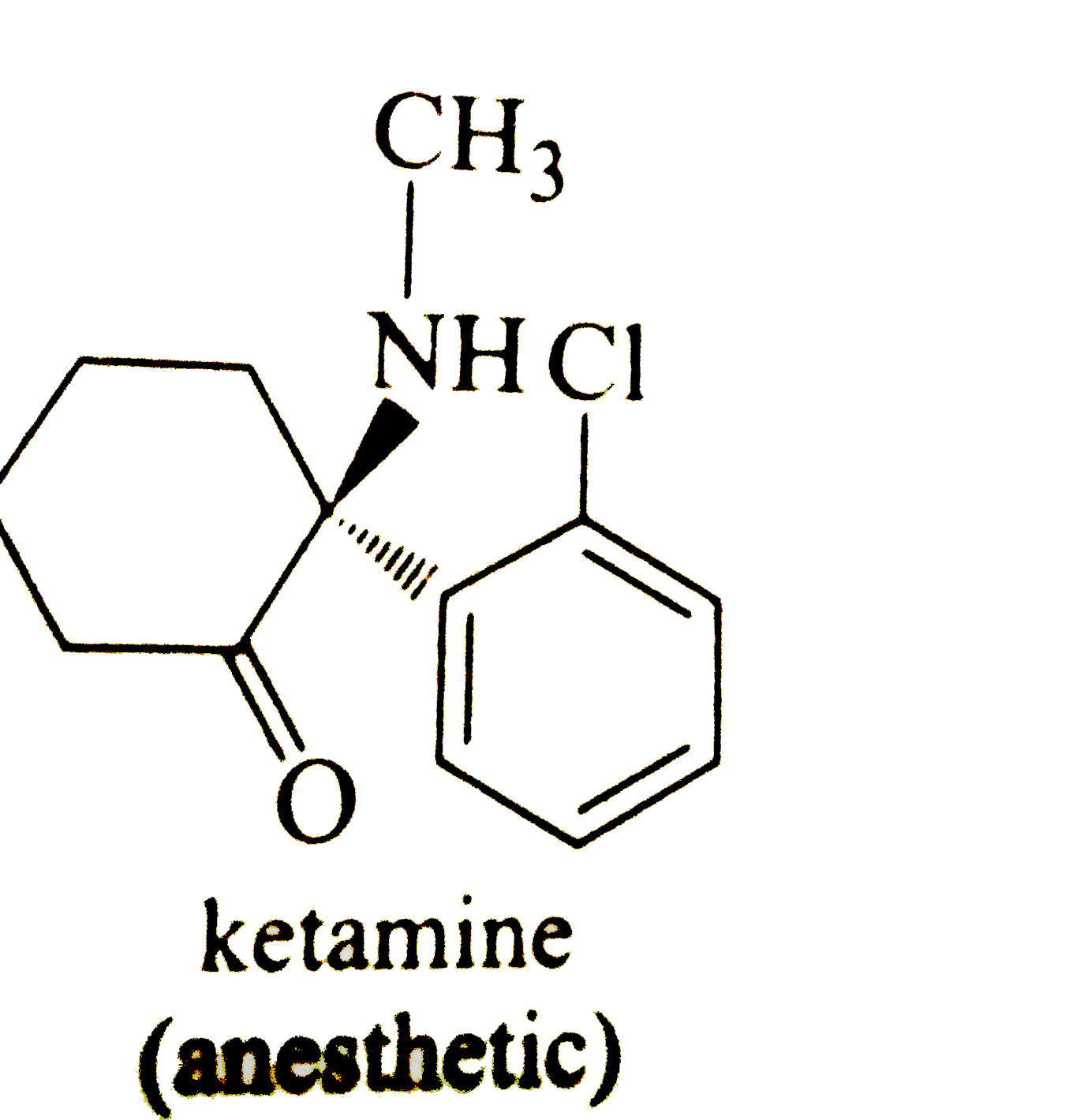
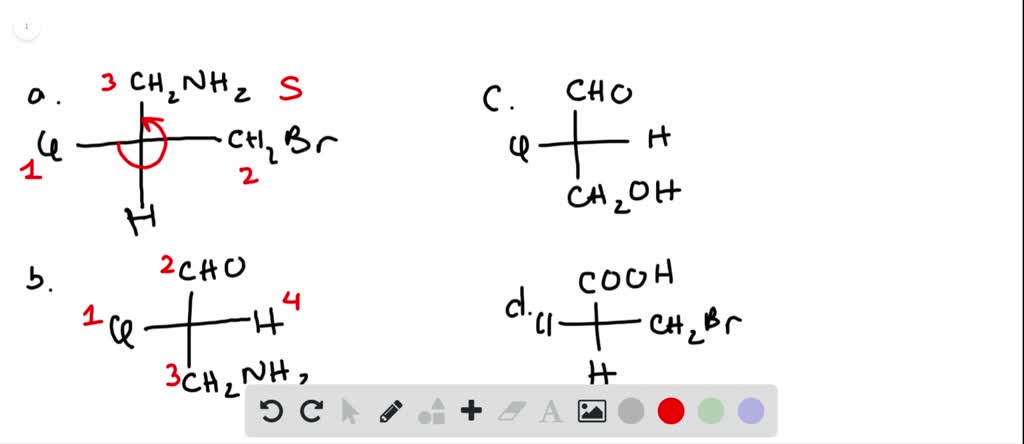
Post a Comment for "45 label each stereogenic center as r or s"